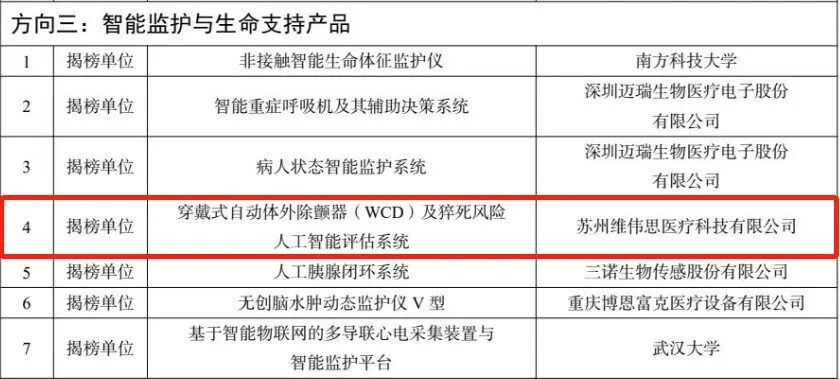
On October 28, 2022, the General Office of the Ministry of Industry and Information Technology and the General and Planning Finance Department of the National Medical Products Administration jointly issued the “Notice on the Announcement of the Shortlisted Units for the Artificial Intelligence Medical Device Innovation Task”. The “Wearable Automatic External Defibrillator (WCD) and Sudden Death Risk Artificial Intelligence Assessment System” project of Weiweisi Medical was successfully selected as the “Take-up Unit” in the category of intelligent monitoring and life support products, becoming the main body of the innovation task.

Demand-based, clinical-oriented
China’s innovation in the field of artificial intelligence
Background introduction
In order to further implement the important instructions of the General Secretary on the work of taking charge of the unveiling, accelerate the deep integration and development of artificial intelligence technology and medical devices, and better serve and protect the life and health of the people, the Ministry of Industry and Information Technology and the National Medical Products Administration jointly carried out the unveiling of the artificial intelligence medical device innovation task. The selection is oriented to the two directions of intelligent products and supporting environment, focusing on eight types of unveiling tasks such as intelligent auxiliary diagnosis products, and soliciting and selecting a group of units with strong innovation capabilities to concentrate on tackling key problems, promote the innovative development of artificial intelligence medical devices, and accelerate the implementation of new technologies and new products.
This selection means that the “wearable automatic external defibrillator (WCD) and sudden death risk artificial intelligence assessment system” created by Vivis Medical meets the requirements of “the unit that announces the smart product category has completed the preliminary research of the product and has a basic finalized product, the product has intellectual property rights and has significant clinical application value”, and has been recognized by the Ministry of Industry and Information Technology and the State Food and Drug Administration.
The WCD product independently developed by Vivis Medical can be effectively used for the risk prevention and ventricular fibrillation treatment of patients at high risk of short-term sudden cardiac death in clinical practice. It has the advantages of simple and convenient wearing, flexible use cycle, reusability and reliable defibrillation effect. WCD makes it possible for people at high risk of sudden cardiac death to live at home, extending the time window for doctors to treat patients.
At present, domestic substitution is gradually being realized in various key sub-tracks. Improving independent innovation capabilities, promoting high-quality development of the industry, redefining international standards and making international layouts have become important missions for domestic medical device companies.
The launch of the wearable automatic external defibrillator independently developed by VIS will break the foreign monopoly and enhance my country’s ability to prevent and treat sudden cardiac death in patients from in-hospital to out-of-hospital.

“Digital Intelligence” Integration
Leading New Trends in the Industry
Looking to the future, VIS Medical, as a potential unicorn enterprise of innovative medical devices at the two levels of Jiangsu Province and Suzhou City, will focus on the application of artificial intelligence technology in sudden death risk assessment through the long-term big data characteristics of wearable products and the advantages of my country’s rapidly developing 5G data transmission. It can greatly extend the patient benefits of WCD products and transform passive defibrillation treatment of sudden cardiac death into active health management with prevention as the main focus.
The innovation and breakthrough of WCD and sudden death risk artificial intelligence assessment system have attracted the attention and attention of relevant national departments:
The wearable automatic external defibrillator was selected into the 2021 Jiangsu Province Key R&D Plan (Social Development)!
Approved by the Medical Device Technology Review Center of the National Medical Products Administration, WCD entered the special review procedure for innovative medical devices
In the future, Vivis Medical will extend the development of the world’s first multi-parameter sudden death risk assessment system through the promotion of ventricular arrhythmia wearable products and remote data service management.
Through this system, Vivis Medical will provide Chinese and global patients with a complete solution for the prevention, monitoring, treatment and rehabilitation of ventricular arrhythmias, leading the transformation of passive treatment of sudden cardiac death into precise treatment combining prevention and treatment.


