On August 16, 2023, the PowerBeat M series portable automatic external defibrillator (Palm AED) developed by Suzhou Weiweisi Medical Co., Ltd. was officially approved by the National Medical Products Administration for registration of Class III active devices. As the first “Palm” AED in China, it is expected to break the current rescue mode of traditional AEDs and usher in a new era of mobile rescue.
In 2021, the group standard “Requirements for the Installation of Automatic External Defibrillators in Public Places” issued by the China Medical Rescue Association recommended that 100 to 200 AEDs should be configured for every 100,000 people. According to the data in the “Analysis and Countermeasures on the Current Status of AED Configuration in China”, around 2019, there were only 2 AEDs per 100,000 people in my country on average, which is in sharp contrast to the configuration of 555 AEDs per 100,000 people in Japan.
The Palm AED weighs only 0.7kg and the front size is only the size of a mobile phone. The traditional AEDs on the market generally weigh 2-3kg. Compared with traditional AEDs, the Palm AED has greatly reduced weight and volume, making it the industry’s first mobile portable AED. With its unique lightweight design, this product is expected to solve the difficulties and pain points of traditional AEDs, such as “small number”, “cannot be found”, “weak supervision”, and “cannot use”.
The traditional “people looking for AED” mode requires the equipment to be within a certain coverage range, because when an accident occurs, the golden rescue time is only 4 minutes. It is undoubtedly quite difficult for rescuers to find a nearby AED and then bring it back to the patient for rescue.
However, the Palm AED uses Weiweisi’s exclusive patented Tiny CombineTM technology. Compared with the traditional fixed AED, the weight is reduced by 2/3 and the volume is reduced by 3/4. It can be directly stored in the rescue bag, realizing dedicated management and participating in the rescue anytime and anywhere, so that “people looking for AED” becomes “AED looking for people”. Rescuers and equipment arrive at the scene at the same time and rescue as soon as possible. This not only greatly increases the survival rate of patients, but also significantly improves the coverage of AED in the future.

In addition, the application of the Palm AED improves the coverage efficiency of unit AEDs, which can effectively solve the problems of insufficient AED configuration and low AED utilization in most cities. The coverage radius of traditional fixed AEDs is about 250 meters, while the Palm AED can not only be carried by patrol personnel, but also carried on mobile transportation. According to actual measurement and comparison, the coverage range of the Palm AED is about 4-36 times that of the fixed AED.
The Palm AED has the characteristics of IP65 dust and water resistance, 1.5m drop resistance, fall resistance, and -40 degrees +70 degrees storage temperature, and can easily adapt to various harsh and complex environmental conditions. The equipment has passed strict environmental stress tests to ensure that it can work normally in various latitudes, temperatures and humidity, altitudes, and various outdoor complex environments in the motherland.
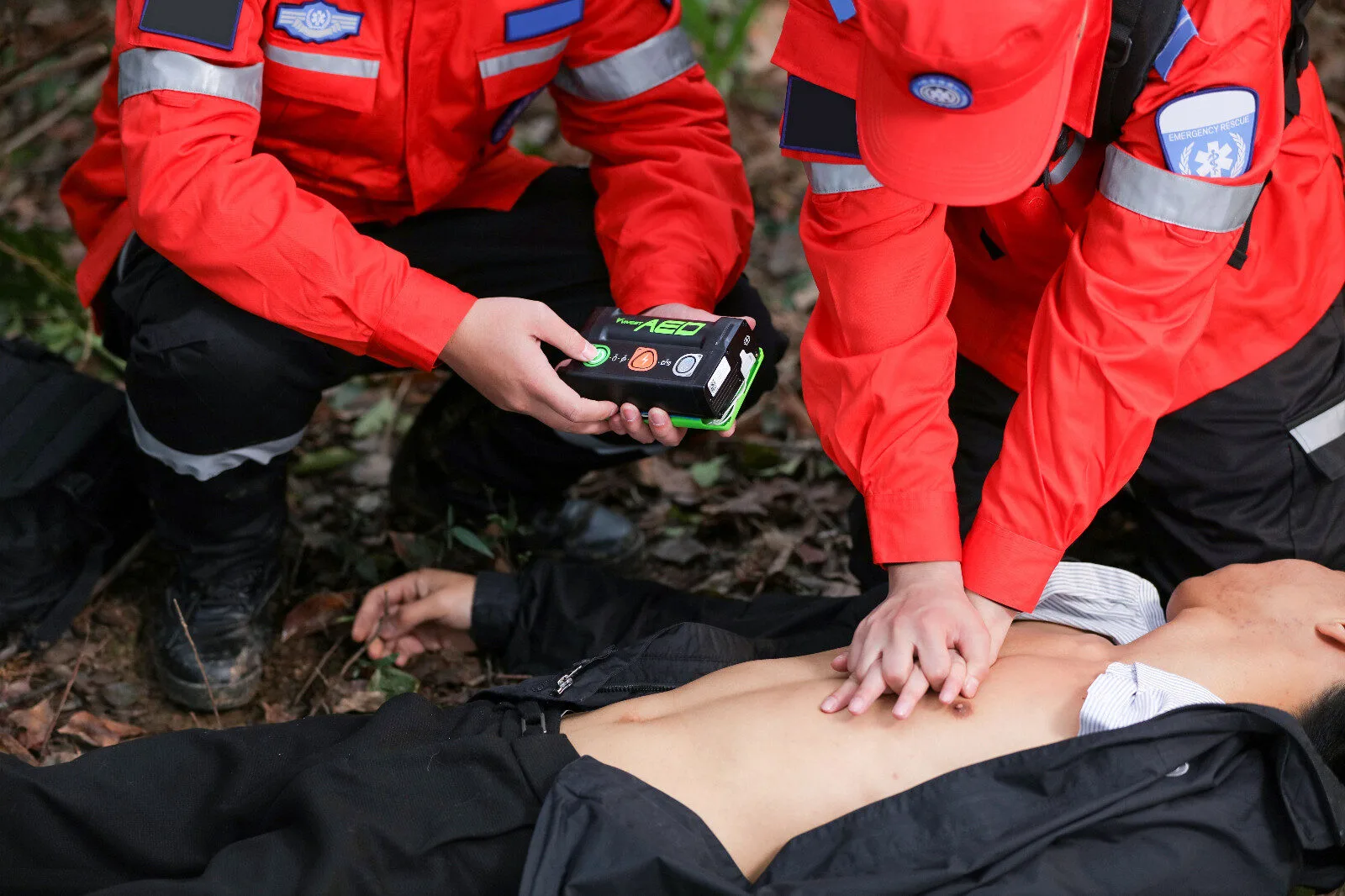
In addition, the Palm AED uses an accurate heart rhythm analysis algorithm, has a wide range of applicability and high accuracy, can automatically and quickly identify defibrillable heart rhythms, and effectively improve the success rate of treatment. The device uses low-energy, biphasic truncated exponential (BTE) defibrillation technology, and only 150J of energy is needed to generate enough transcardiac current for defibrillation treatment. The discharge parameters can be automatically adjusted according to the different transthoracic impedances of different patients, that is, each electric shock is automatically optimized from the first rhythm analysis, providing individualized treatment for each patient. While efficiently defibrillating, it effectively reduces damage to the patient’s myocardium.
Weiweisi is an innovative medical technology company focusing on ventricular arrhythmia prevention and treatment and rhythm data services. AED products are exported to dozens of countries and regions around the world. The company has always been committed to providing the world’s leading full-process complete solutions for ventricular arrhythmia prevention, monitoring, treatment, and rehabilitation. Its products have entered the national innovation green channel and have been approved by the Ministry of Industry and Information Technology and the State Food and Drug Administration. It has been successfully selected as the unit for the innovation task of artificial intelligence medical devices and has become the main body of innovation task research.
In the future, miniaturization, portability, and low-energy defibrillation of AED equipment will become the main development trend. Vivis hopes to open a new era of medical technology innovation through this “palm-sized treasure” AED, help improve the efficiency and survival rate of out-of-hospital emergency treatment, and promote the all-round layout of domestic AED. As a national science and technology medical brand, Vivis also has the responsibility and obligation to bring continuously optimized AED innovative products and solutions to the society and patients.
Let every heart beat healthily!


