Our History
- Home
- Our History
Since
2019
At ViVest, our history is a testament to perseverance, innovation, and an unwavering commitment to excellence. From our humble beginnings to becoming a global leader in cardiac health technology, every milestone reflects our dedication to advancing life-saving solutions. As we continue to push the boundaries of innovation, we remain focused on safeguarding lives and improving healthcare worldwide.
Our Journey

2019/03: A Vision is Born
ViVest was officially founded in Suzhou Industrial Park. Our mission to prevent and treat ventricular arrhythmias began in a modest office, driven by a dedicated team of innovators.
2019/09: Building the Foundation
The establishment of our Shenzhen R&D Center sets the stage for groundbreaking research and the development of advanced medical devices.
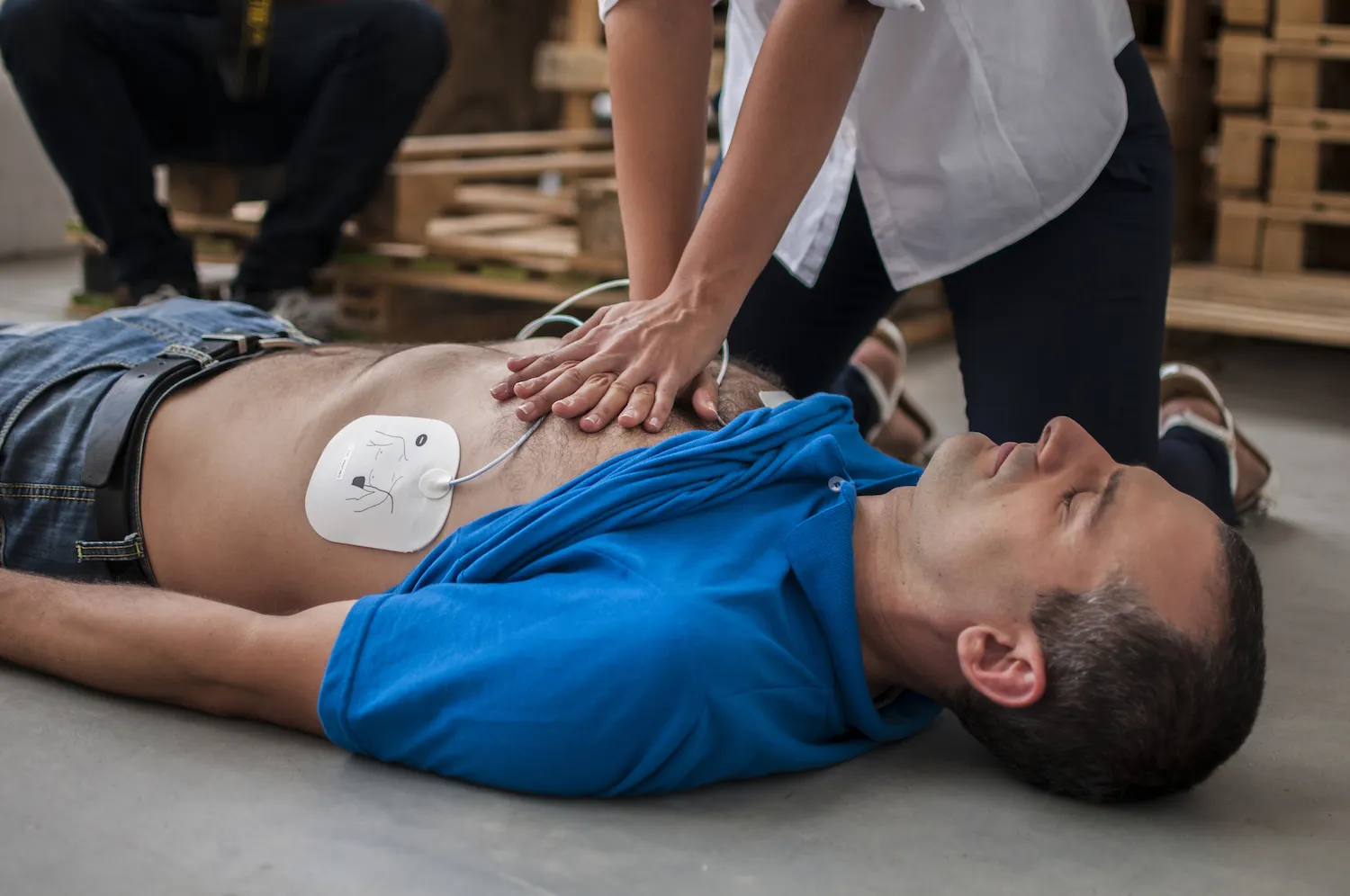
2020/06: Advancing through Algorithms
Our Beijing Algorithm Center is launched, empowering us to harness cutting-edge data and algorithms to drive innovation in medical solutions.
2020/12: Pioneering Wearable Technology
The Wearable Cardioverter Defibrillator enters the special review process for innovative devices, reflecting our commitment to transforming cardiac care with wearable technologies.

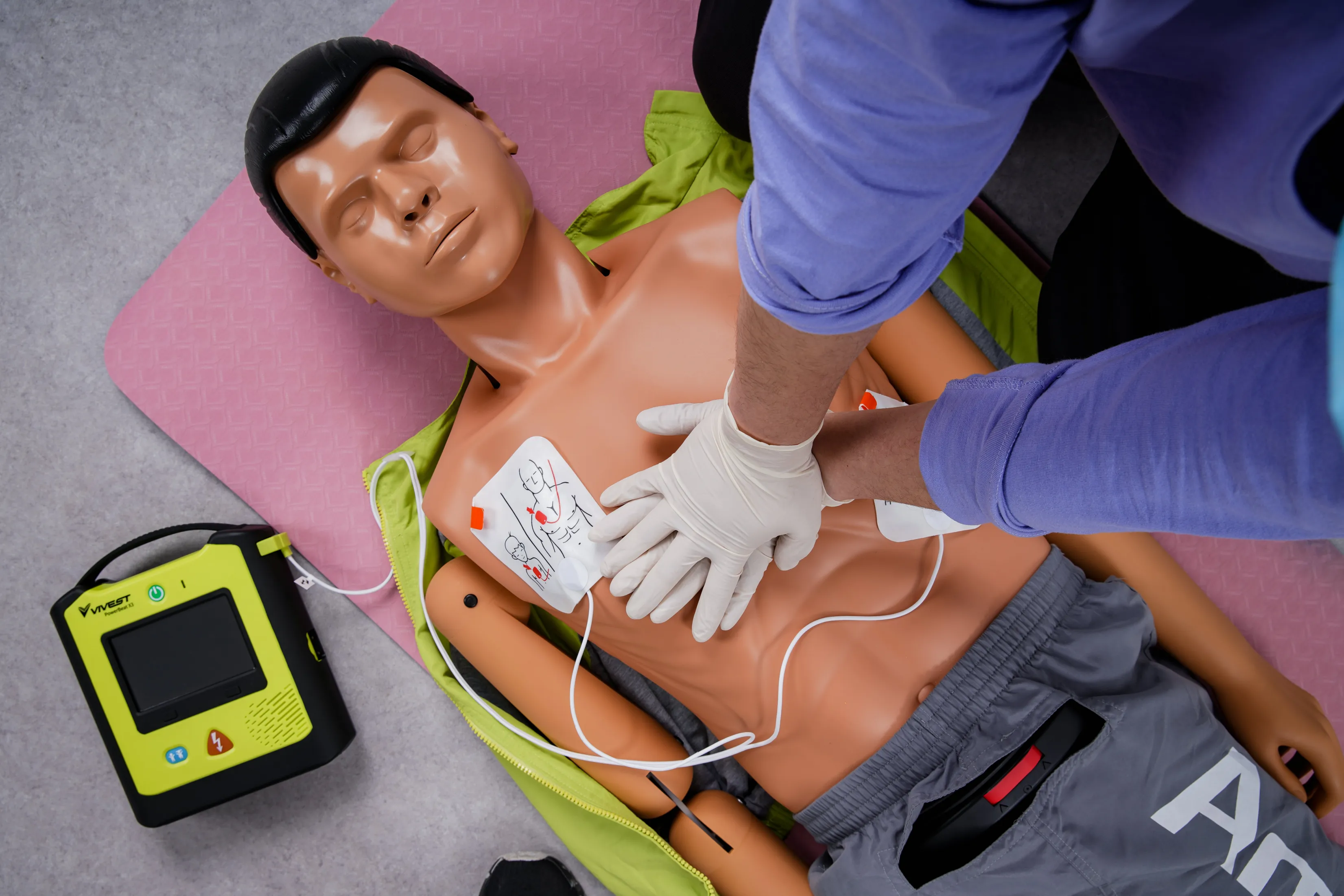
2021/04: Achieving Global Standards
PowerBeat X Series AED passes CE certification, and our Suzhou factory achieves ISO13485 certification, ensuring the highest quality in manufacturing.
2021/09: Recognized for Excellence
ViVest is awarded “High-tech Enterprise” status, while the PowerBeat X Series AED receives the prestigious NMPA Class III medical device certificate.


2023/08: Strengthening Our Impact
The PowerBeat M Series AED earns the NMPA Class III medical device certificate, further solidifying our position in the global medical device market.
2023/11: Expanding Our Reach
We move into a state-of-the-art 5,000-square-meter headquarters, a major milestone in supporting our global operations and future growth.


2025/02: Achieving CE-MDR Compliance
Our X-Series AEDs (X1 & X3) achieve CE-MDR compliance, certified by TÜV SÜD, enabling us to offer these advanced devices across Europe, reaffirming our commitment to quality and reliability.
What do our employees say?
Working at ViVest makes me feel truly fulfilled. The company focuses on innovation and provides a broad research and development platform, allowing me to constantly challenge myself and improve my professional skills. The team atmosphere is also highly collaborative, and everyone works together to solve problems, which makes me feel deeply involved. Working here not only helps me grow personally, but also enables me to make a meaningful contribution to society.

Mrs. Christina
R&D engineer
ViVest has provided me with a fast-paced platform for professional growth. Here, I’ve had the opportunity to contribute to the global advancement of heart health solutions. The company encourages independent thinking and innovation, which has helped me continuously strengthen my professional skills and access more development opportunities. ViVest not only prioritizes business growth, but also places great emphasis on the personal well-being and career development of its employees.

Li Na
Marketing manager


